Explore streak, hardness, cleavage, fracture, density, and magnetism in mineral identification
For the identification and characterization of mineral specimens, their morphology, physical and chemical properties, and characteristics are generally considered.
Morphology of Minerals
1. Morphology of Individual Minerals
Individual minerals can exhibit various forms. Some minerals elongate in one direction, forming columnar or needle-like shapes. Others extend in two directions, resulting in platy or tabular forms. Some minerals have equal lengths in three directions and can form cubic or octahedral shapes.
Apart from single-crystal growth, crystals can also be twinned, where two or more crystals of the same kind grow together in a specific orientation. This twinning produces characteristic forms, such as gypsum’s swallowtail twins (Figure 1-1A) or staurolite’s cruciform twins (Figure 1-1B).
2. Morphology of Aggregate Minerals
Aggregates are composed of individual mineral units. Each mineral aggregate often exhibits a habitual form. If a mineral elongates in one direction, its aggregate is often fibrous or needle-like.
If it extends in two directions, the aggregate tends to be platy or scaly. If it has equal lengths in three directions, the aggregate appears granular (when the grain boundaries are visible to the naked eye) or massive (when the grain boundaries are not discernible). Within the massive aggregates, denser ones are referred to as compact, while looser ones are called earthy. Additionally, there are some special forms of aggregates, such as:
Radiating: Long columnar or needle-like minerals arranged radially from a central point, as seen in rhodochrosite (Figure 1-1C).
Druse: Complete crystal clusters grown in rock fissures or cavities, like quartz or calcite druses.
Oolitic and pisolitic: Aggregates composed of spherical minerals. Oolitic aggregates have concentric layers resembling fish eggs, while pisolitic aggregates are bean-shaped.
Stalactitic: Resembling ice cones formed under eaves in winter, with circular cross-sections and internal concentric layering, sometimes exhibiting radial structures.
Botryoidal and reniform: Grape-like forms are called botryoidal, and kidney-like forms are called reniform. Both exhibit internal concentric or radial structures.
Nodular: Irregularly shaped spherical or ellipsoidal bodies with internal concentric or radial structures.
Physical Properties of Minerals
The physical properties of minerals include optical properties, mechanical properties, magnetism, and electrical conductivity. Optical properties refer to how minerals behave under visible light, including transparency, luster, color, and streak. Mechanical properties describe how minerals respond to external forces, such as hardness, cleavage, and fracture.
1. Transparency:
Refers to the ability of a mineral to transmit visible light. Minerals that allow light to pass through thin sections (0.03 mm thick) are called transparent, while those that do not are called opaque.
2. Luster
Luster refers to the ability of minerals to reflect visible light. It can be classified based on the strength of reflection as follows:
Metallic luster: Strong reflection similar to the reflection of light on a chrome-plated metal surface, such as the luster of galena.
Submetallic luster: Moderate reflection resembling the reflection of typical metals, like the luster of magnetite.
Non-metallic luster: Further divided into adamantine luster and vitreous luster based on their reflective characteristics:(1)Adamantine luster: Strong and dazzling reflection, like the luster of a diamond.(2)Vitreous luster: Relatively weak reflection resembling the reflection on a glass surface, such as the luster of calcite or quartz crystal faces. The mentioned lusters refer to the luster exhibited by fresh minerals on flat crystal faces, cleavage surfaces, or polished surfaces. If the mineral surface is not flat or forms aggregates, the luster may weaken or exhibit specific lusters like greasy luster or earthy luster.
3. Color
Color of an object usually refers to the color it displays under white light. In mineralogy, colors can be categorized into three types: intrinsic color, extrinsic color, and false color.
Intrinsic color: Refers to the color directly related to the mineral’s inherent chemical composition. It is relatively stable and characteristic for a specific mineral, such as the cherry-red color of hematite.
Extrinsic color: Refers to colors caused by factors other than the mineral’s inherent composition. It can be caused by minor mechanical impurities mixed within the mineral, substitution of trace impurity elements during solid solution processes, or the presence of certain crystal lattice defects within the mineral. For example, pure quartz is colorless, but it can exhibit various colors like purple (due to the presence of trivalent iron) when containing trace impurity elements.
False color: Refers to coloration caused by physical optical processes such as interference and diffraction of light. False colors can be observed on mineral surfaces due to the formation of oxide films.
4. Streak
Streak refers to the color of a mineral’s powder. It is particularly important for identifying certain metallic minerals. For instance, pyrite has a greenish-black streak, while hematite exhibits red, black, or steel-gray colors but always has a reddish streak. The streak of transparent minerals is usually white or nearly white and does not have diagnostic significance.
5. Hardness
Hardness refers to a mineral’s resistance to external forces. In visual identification, it primarily indicates the mineral’s ability to resist scratching. Hardness is primarily determined by the strength of atomic, ionic, or molecular bonds within the mineral. The Mohs scale of hardness is commonly used as a standard for measurement. The Mohs scale consists of 10 minerals with varying hardness (Table 2-1). Talc has the lowest hardness of 1, while diamond has the highest hardness of 10. To determine the hardness of a mineral, it is compared to the standard minerals in the hardness scale by attempting to scratch them. For example, if a mineral can scratch calcite but can be scratched by fluorite, its hardness would be between 3 and 4.
Table 2-1: Mohs Scale of Hardness
| Hardness Level | Represented Mineral | Hardness Level | Represented Mineral |
| 1 | Talc | 6 | Orthoclase Feldspar |
| 2 | Gypsum | 7 | Quartz |
| 3 | Calcite | 8 | Topaz |
| 4 | Fluorite | 9 | Corundum |
| 5 | Apatite | 10 | Diamond |
Other commonly encountered objects can also be used as substitutes for the minerals in the hardness scale. For example, the hardness of a fingernail is around 2-2.5, a copper key is 3, a small steel knife is approximately 5-5.5, and window glass is 6.
The Mohs scale of hardness can only determine the relative hardness of different minerals and cannot provide absolute values. For instance, talc has a hardness of 1, while quartz has a hardness of 7, but this does not mean quartz is seven times harder than talc. In terms of measured hardness values, talc is approximately 2.3, and quartz is around 300, making the latter roughly 130 times harder than the former.
6. Cleavage
Cleavage refers to the ability of a crystal to split along specific crystalline directions when subjected to external forces, resulting in planar surfaces called cleavage planes. This property is determined by the internal structure of the crystal. Since the distances between atoms, ions, or molecules are not equal in different directions within the crystal, the strength of attraction between them varies. Cleavage planes always occur along the directions where the interplanar forces are weakest. The plane with the highest density and greatest distance between interplanar points has the weakest bonding, making it susceptible to cleavage (Figure 2-2).
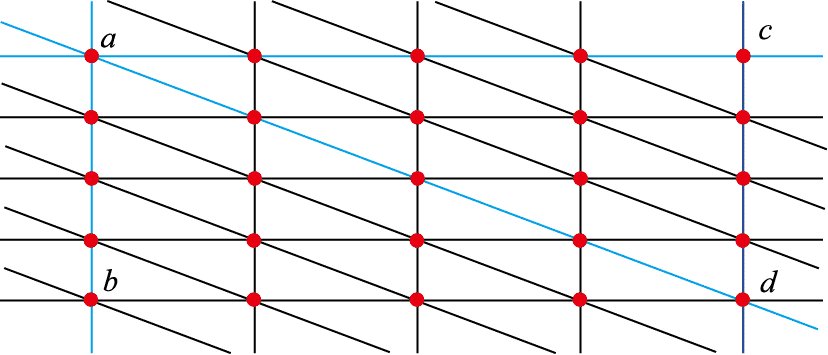
Figure 2-2: Possible cleavage directions along interplanar points.
The figure shows three interplanar directions perpendicular to the plane: ab, ac, and ad. Among these directions, the interplanar points along the ab direction have the highest density and the greatest distance, making cleavage most likely to occur along this direction.
Certain minerals have weak bonding in several directions, leading to the formation of multiple cleavage planes, as seen in calcite. Minerals bonded by metallic bonds, such as native gold and native copper, have dispersed free electrons within their structures. When these minerals are subjected to external forces, only internal lattice sliding occurs, and they do not cleave in fixed directions. Instead, they exhibit high ductility without cleavage.
The directions and degree of cleavage vary among different minerals. Based on the cleavage’s perfection, it can be categorized into four levels: perfect, good, fair, and poor. Cleavage is an essential characteristic for identifying minerals. For example, mica exhibits perfect cleavage in one direction, making it easy to split into thin sheets along this direction. Calcite has three directions of perfect cleavage, resulting in fractures along these three directions, forming a series of rhombohedral-shaped fragments.
7. Fracture
Fracture refers to the way a mineral breaks when subjected to external forces without cleaving along fixed crystallographic directions. Fracture surfaces have no specific pattern, and the fracture’s morphology can vary, such as shell-like, irregular, jagged, or flat. Fracture is mainly observed in minerals with underdeveloped cleavage or mineral aggregates, like quartz.
8. Density
For non-metallic minerals, density can be classified into three levels: light, with a density below 2.5 g/cm3, perceived as light when held in hand; medium, with a density between 2.5 and 4 g/cm3, perceived as moderately heavy or average weight when held in hand; heavy, with a density greater than 4.0 g/cm3, perceived as very heavy when held in hand.
9. Magnetism
Magnetism is also an important identifying characteristic of certain minerals. For example, magnetite and pyrrhotite are attracted to a common magnet, while native bismuth is repelled by a magnet.